Despite the high prevalence of HBV & HCV, and the availability of effective curative treatment for HCV infection, as well as long-term suppressive antiviral treatment for HBV; most people infected with HBV or HCV globally have never been tested and so remain unaware of their infection. Key reasons for this current very low rate of hepatitis testing in LMICs include: limited laboratory capacity and access to reliable, HCV diagnostics, and lack of testing guidance to the healthcare providers working in the laboratories.
The main objective of the training program for laboratory professionals is to inform feasibility of potential recommendations on testing approaches (who and where to test) and how to test (selection of assays) in the viral hepatitis testing guidelines, and also to assess key perceived barriers/challenges and strategies to address these and so guide implementation of hepatitis testing and treatment services.
The program is conducted under aegis of Dr. Ekta Gupta, Professor, Clinical Virology, ILBS with support from Dr. Reshu Agarwal, Associate Professor, Clinical Virology, ILBS, Senior Residents and technical staff.

| DETAILS | TOPIC AND DESCRIPTION |
| Overview and laboratory diagnosis of viral hepatitis | Introduction to the program & Pre-training assessment |
| Overview of viral hepatitisOverview of hepatitis viruses; current scenario of testing in India; WHO & NVHCP guidelines on testing; natural history and diagnostic approaches; interpretation of screening in HBV, HCV & HEV; current testing methods; sampling innovations & CBNAAT. | |
| Serological methods in viral hepatitis testing and quality controlDirect vs indirect methods; definition & principles; advantages & disadvantages. Quality control and its functional phases; caliberators vs controls; types of charts in QC | |
| Molecular methods in viral hepatitis testingDNA structure; types of extractions; PCR cycle; types of molecular diagnostic tests | |
| Bio-safety & Infection Prevention | Good laboratory practicesInfrastructure; Personnel, Training & Development; Equipment’s; Reagents & Materials; Specimen Collection; Requisition Form; Accession List; Testing & Reporting of Results; Data Management; SOPs; Quality Assurance Programme; Safety in Laboratories. |
| Needle Stick Injury & Post Exposure ProphylaxisWho is at risk; factors influencing NSI; occupational exposure; NSI do’s and don’ts; reporting of NSI and PEP for HIV, HBV & HCV. | |
| Live demonstration & hands on training | RCT – Rapid Card Tests |
| ELISA – enzyme-linked immunoassay | |
| CLIA – ChemiLuminescence Immuno Assay | |
| Micro pipetting techniques and calibration | |
| Closing | Post-test, feedback and certificate distribution |
There is a huge burden to undetected/untested HBV & HCV cases across the globe. The global burden of viral hepatitis B & C accounts for over 300 million cases. Apart from this, there is a huge gap as majority of people are unaware of their infection. Over the years, new advances have been made in rapid testing of HBV and HCV. These new technological advances can help in early detection of the infection leading to early treatment in HBV & HCV patients but lack of information leads to poor diagnosis of viral hepatitis.
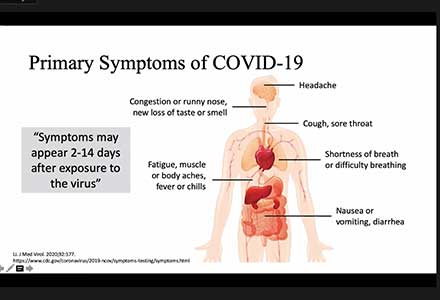
The acceptance and awareness of diagnostic measures to detect and contain COVID-19, leverages an opportunity to amplify awareness of viral hepatitis to the same level as that of HIV and COVID-19 to improve health status. It may take a pandemic to catalyse awareness and increase testing for HBV & HCV.
Owing to the speculations and uncertainty around the management of COVID-19 and viral hepatitis, project PRAKASH, initiated a two days virtual Hepatitis Update Program addressing the laboratory diagnosis of both the conditions.
The program is conducted under aegis of Dr. Ekta Gupta, Professor, Clinical Virology, ILBS with support from Dr. Reshu Agarwal, Associate Professor, Clinical Virology, ILBS, Senior Residents and technical staff.
| TOPIC & DESCRIPTION | SPEAKER |
| Introduction to the program & Pre-training assessment | Ms. Akanksha Bansal |
| Overview of viral hepatitis (A-E) Overview of hepatitis viruses; current scenario of testing in India; WHO guidelines on testing; natural history and diagnostic approaches; interpretation of screening in HBV, HCV & HEV | Dr. Ekta Gupta |
| Serological methods in viral hepatitis testing and quality control Direct vs indirect methods; definition & principles; advantages & disadvantages; ELISA & RDT techniques. Quality control and its functional phases; caliberators vs controls; accuracy and precision; mean, median and standard deviation; Levey jenning charts and westgard rules in quality control. | Dr. Reshu Agarwal |
| Molecular methods in viral hepatitis testing DNA structure; types of extractions; PCR cycle; types of molecular diagnostic tests; unidirectional workflow; newer techniques in molecular testing – qualitative vs quantitative testing. | Dr. Arjun Bhugra |
| Needle Stick Injury & Post Exposure Prophylaxis Who is at risk; factors influencing NSI; occupational exposure; NSI do’s and don’ts; reporting of NSI and PEP for HIV, HBV & HCV; monitoring of exposed; testing of source and prevention of NSI. | Dr. Reshu Agarwal |
| DAY 2 | |
| Overview of COVID-19 SARS CoV-2 Virus: origin, timeline; Basic Virology; Replication; Pathogenesis; Transmission; SARS CoV-2 variants and spread. | Dr. Ekta Gupta |
| Laboratory diagnosis of COVID-19 Epidemiological surveillance & diagnosis of active infection; ICMR advisory on testing; specimen, collection & transport of samples; direct & indirect testing modalities; antigen and antibody testing; serological assays and testings. | Dr. Reshu Agarwal |
| Bio – safety and bio medical waste management in COVID-19 Donning & doffing of PPE; bio-safety during sample collection & transport; bio-safety levels; BMW categories, segregation and treatment. | Dr. Reshu Agarwal |
| Good laboratory practices and clinical virology Infrastructure; Personnel, Training & Development; Equipment’s; Reagents & Materials; Specimen Collection; Requisition Form; Accession List; Testing & Reporting of Results; Data Management; SOPs; Quality Assurance Programme; Safety in Laboratories. | Dr. Rahul Garg |
| Post test & feedback | Project TEAM |


Call us now

Drop us an email
Monday to Saturday
